Clinical Results
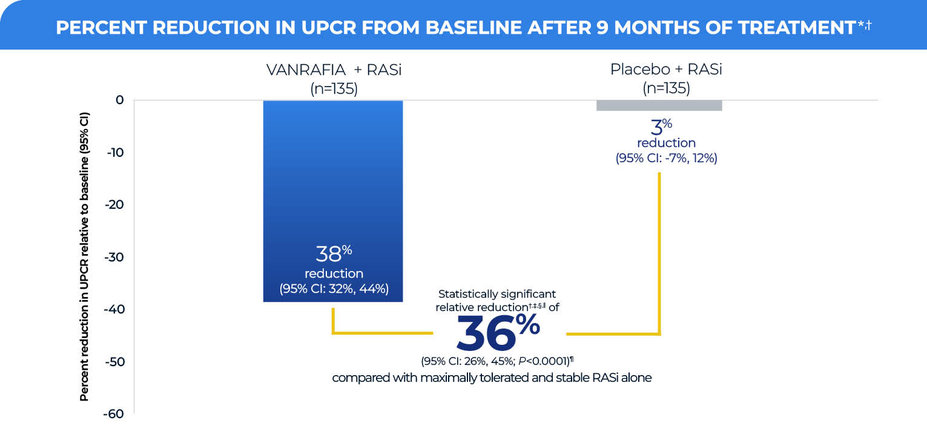
When added to a maximally tolerated and stable RASi regimen,
VANRAFIA delivered a significant reduction in UPCR at Week 36 for patients with IgAN in the main cohort (n=270)1,2
*LS geometric mean ratio in UPCR (sampled from a 24-hour urine collection) to baseline was reported as a percent reduction along with the respective 95% CI.
†MMRM analysis included all observed UPCR data except for subjects with intercurrent events (eg, restricted medication use, chronic dialysis, kidney transplant). These subjects had UPCR data excluded beginning at the start date of the earliest event. The only intercurrent events observed were restricted medication use, which occurred in 3.0% and 5.2% of VANRAFIA- and placebo-treated subjects, respectively.
‡Statistically significant results coming from an interim analysis for accelerated approval through 36 weeks of treatment. The study for full approval is ongoing and will be based on data from 132 weeks of treatment.
§The estimate of the ratio of LS geometric mean ratio in UPCR (sampled from a 24-hour urine collection) to baseline comparing VANRAFIA with placebo was reported as a relative percent reduction along with the respective 95% CI and 2-sided P value.
IIThe relative percent difference between VANRAFIA and placebo is equal to the ratio of the geometric mean minus 1 multiplied by 100: 100*[(0.62/0.97)-1]=-36%.
¶Two-sided P value statistically significant at the 0.01 level.
Patients in both the VANRAFIA and placebo groups were on a maximally tolerated and stable dose of RASi at baseline that was continued throughout the duration of the study
The treatment effect on UPCR at Week 36 was consistent across subgroups, including age, sex, race, and baseline disease characteristics (such as eGFR and proteinuria levels), within the main cohort
In an exploratory analysis, a similar treatment effect on 24-hour UPCR was observed in an exploratory cohort of patients on an existing RASi + SGLT2i regimen
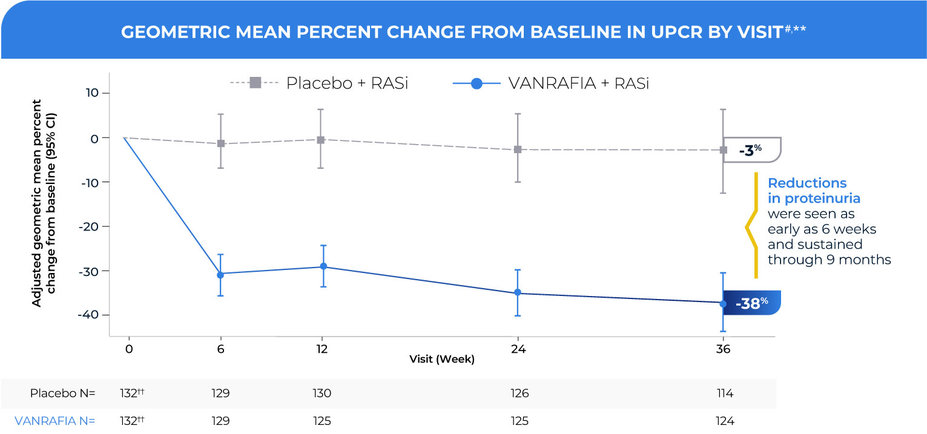
When added to a maximally tolerated and stable RASi regimen,
VANRAFIA delivered rapid and sustained proteinuria reduction through 9 months of treatment1,2
#Adjusted percent change relative to baseline in UPCR (sampled from a 24-hour urine collection) was estimated based on the MMRM analysis in Table 2 of the VANRAFIA Prescribing Information. N represents the number of evaluable subjects included in the analysis (ie, with nonmissing UPCR values and baseline covariates and did not have restricted medication use, or chronic dialysis, or kidney transplant) for each visit and treatment group.
**Values reported in the figure were expressed as percent change from baseline and 95% CI, estimated from the regression model in Table 2 of the VANRAFIA Prescribing Information.
††A total of 3 patients in each group had no postbaseline data for the urinary protein-to-creatinine ratio; these patients were excluded from the number of patients at baseline.
Patients in both the VANRAFIA and placebo groups were on a maximally tolerated and stable dose of RASi at baseline that was continued throughout the duration of the study
The treatment effect on UPCR at Week 36 was consistent across subgroups, including age, sex, race, and baseline disease characteristics (such as eGFR and proteinuria levels), within the main cohort
In an exploratory analysis, a similar treatment effect on 24-hour UPCR was observed in an exploratory cohort of patients on an existing RASi + SGLT2i regimen
VANRAFIA is indicated to reduce persistent proteinuria in adults with primary IgAN at risk of rapid disease progression, generally a UPCR ≥1.5 g/g
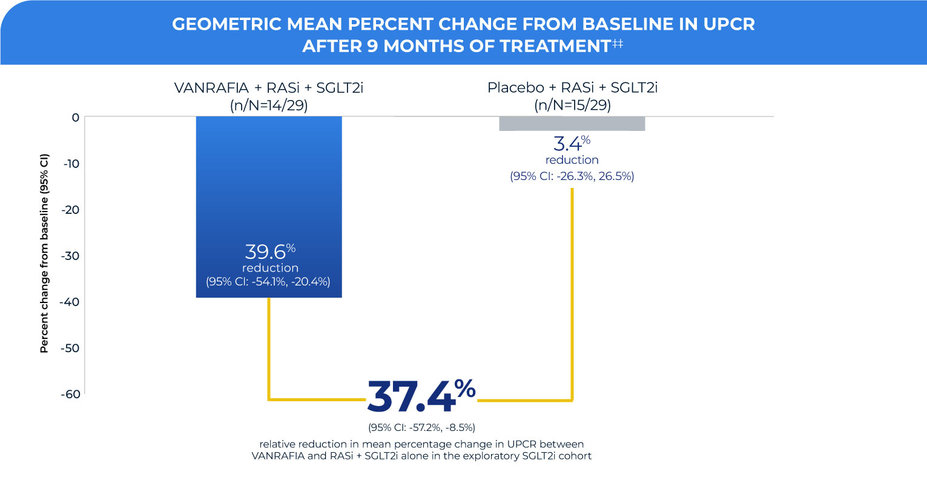
In an exploratory analysis of the ALIGN study,
A similar treatment effect on UPCR was seen in patients (N=29) treated with an optimized dose of RASi + SGLT2i prior to randomization1,2
‡‡Based on a repeated measures analysis with the change from baseline of natural log UPCR at each post baseline timepoint as outcomes; UPCR values are censored (excluded) for subjects with intercurrent events (eg, restricted medication use, chronic dialysis, kidney transplant) beginning at the start date of the earliest event. Missing UPCR values implicitly imputed assuming missing at random.
Patients in both the VANRAFIA and placebo groups were on a maximally tolerated and stable dose of RASi + SGLT2i at baseline that was continued throughout the duration of the study
Limitations: In ALIGN, UPCR was observed in an exploratory cohort of patients on RASi + SGLT2i treatment at study start. Graph shows UPCR in this cohort at Month 9. No clinical or statistical conclusions can be drawn. Results cannot be generalized to patients with total urine protein <1 g/day. Underrepresentation of Black patients limits generalizability to these patients
Curious about the VANRAFIA safety profile?
Add once-daily VANRAFIA