Trial Design
ALIGN: A phase 3 study assessing VANRAFIA in combination with a maximally tolerated and stable dose of RASi in IgAN1-3
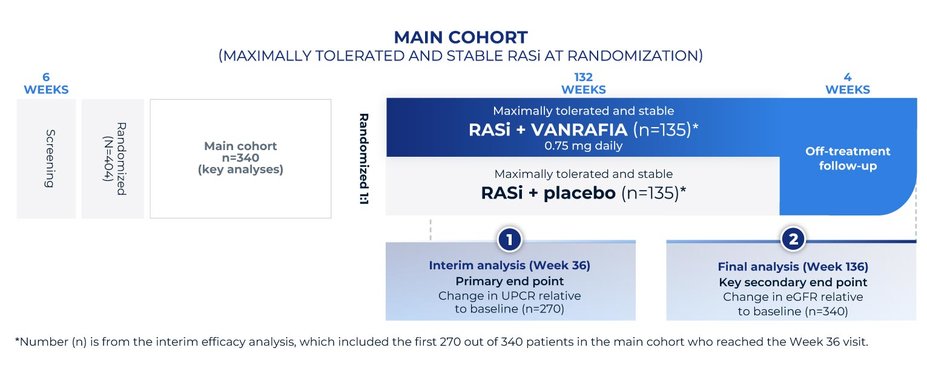
Study design: Phase 3, global, multicenter, randomized, double-blind, placebo-controlled study
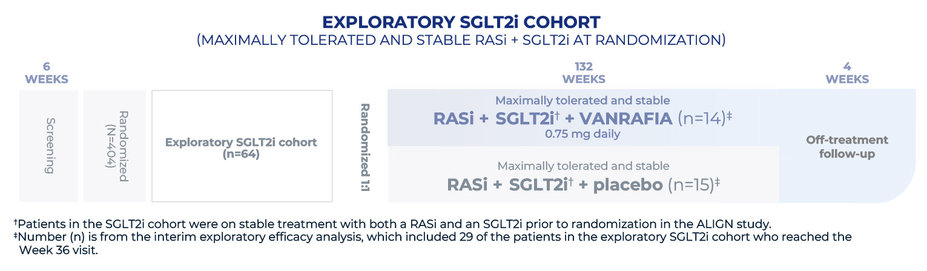
Study population: 340 adult patients with biopsy-proven primary IgAN, urine protein ≥1 g/day, and an eGFR of ≥30 mL/min/1.73 m2 on a maximally tolerated and stable dose of RASi. An additional exploratory SGLT2i cohort of 64 patients who were also on a stable dose of an SGLT2i at baseline was enrolled. The interim efficacy analysis was based on the first 270 patients in the main cohort who reached the Week 36 visit
Supportive care (RASi): Patients were primarily treated with a stable dose of maximally tolerated RASi therapy. In a separate exploratory cohort, 64 patients received an SGLT2i in addition to RASi therapy
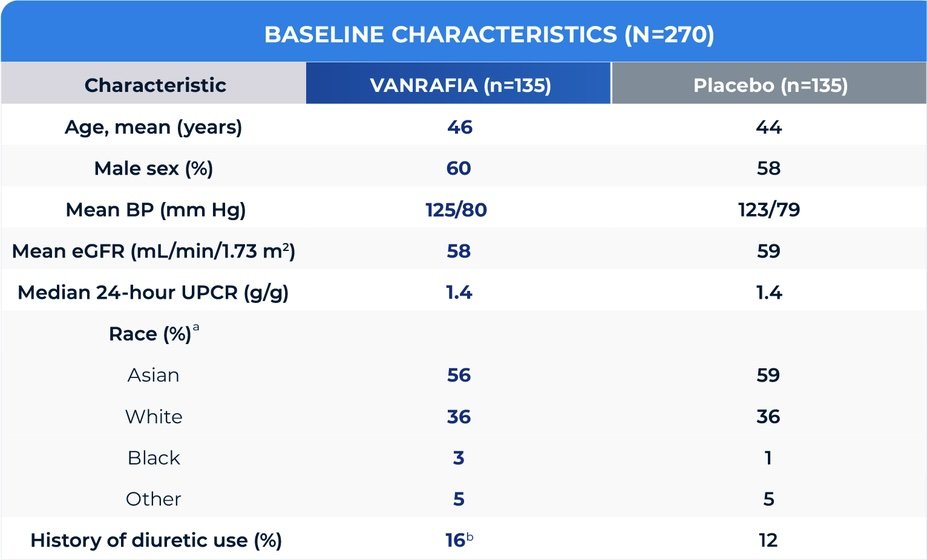
aRace was reported by the patients. “Other” includes patients with multiple races and no races reported.
bThe percentage was calculated on the basis of the safety population.
SELECTED KEY INCLUSION CRITERIA
Adults ≥18 years of age
Biopsy-proven primary IgAN
Maximally tolerated and optimized RASi therapy (ACEi or ARB) regimen for at least 12 weeks
Urine protein ≥1 g/day
eGFR ≥30 mL/min/1.73 m2
Exploratory SGLT2i cohort only: On a stable dose of an SGLT2i plus a maximally tolerated and optimized dose of a RASi
SELECTED KEY EXCLUSION CRITERIA
Current diagnosis with another chronic kidney disease, including diabetic kidney disease or another primary glomerulopathy
Clinical suspicion of rapidly progressive glomerulonephritis or Henoch-Schönlein purpura (IgA vasculitis)
Clinical diagnosis of nephrotic syndrome
BNP >200 pg/mL or Hb below 9 g/dL at screening
History of organ transplantation (apart from corneal transplant)
Use of systemic immunosuppressant medicationsc for >2 weeks in the past 3 months. Use of rituximab within the past 6 months
Received an investigational agent within 1 month
Known history of clinically significant liver disease or transaminase or bilirubin values more than twice the upper limit of normal
cIncluding systemic corticosteroids (eg, prednisone, prednisolone, nefecon), mycophenolate, azathioprine, cyclosporine, tacrolimus, etc; or use of herbs such as Tripterygium wilfordii Hook F, Caulis sinomenii, and Sinomenium acutum.
VANRAFIA was studied in patients with IgAN who were on a maximally tolerated and stable dose of RASi1,2
ACCELERATED APPROVAL
VANRAFIA was approved by the FDA based on UPCR data from the interim analysis at 36 weeks. It has not been established whether VANRAFIA slows kidney function decline in patients with IgAN.
MAIN EFFICACY ANALYSIS
The efficacy analysis included the first 270 out of 340 patients in the main cohort who reached the Week 36 visit. This analysis only included patients on prior therapy with a RASi. This analysis did not include the exploratory SGLT2i cohort.
SAFETY ANALYSIS
The safety analysis included patients from both cohorts (n=403)§ for the duration that they received VANRAFIA. This analysis included patients on prior therapy with a RASi with or without an SGLT2i.
§A total of 404 patients were randomized for the safety analysis; 403 were included.
Learn more about the clinical results from the ALIGN trial
Curious about the VANRAFIA safety profile?